SillaJen, GLPharmTech. Hit Limit Up; Inventage Lab Swings from Plunge to Surge on Obesity Drug Da...
등록 2025-04-24 오전 8:14:14
팜이데일리 프리미엄 기사를 무단 전재·유포하는 행위는 불법이며 형사 처벌 대상입니다.
이에 대해 팜이데일리는 무관용 원칙을 적용해 강력히 대응합니다.
이에 대해 팜이데일리는 무관용 원칙을 적용해 강력히 대응합니다.
이 기사는 2025년4월24일 8시14분에 팜이데일리 프리미엄 콘텐츠로 선공개 되었습니다.
구독하기
[Kim Saemi, Edaily Reporter] In South Korea’s biotech sector on 23 April, shares of SillaJen, Inc. and GLPharmTech. hit the daily upper limit. SillaJen extended its rally for a second straight session on multiple bullish catalysts. Meanwhile, Inventage Lab, which had plunged during regular trading, reversed sharply after-hours to also reach the limit up, boosted by promising data on its oral obesity treatment.
SillaJen, Inc. Hits Limit Up for Second Day on Multiple Catalysts
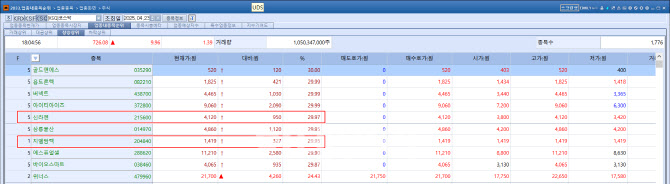
According to KG Zeroin’s MP Doctor platform (formerly Market Point), shares of SillaJen surged 950 won, or 29.97%, to close at 4,120 won. This followed a 29.92% jump the previous session, marking two consecutive limit-up days. The stock has now risen for ten straight trading sessions.
The rally was driven by several positive developments: anticipated benefits from the FDA’s plan to phase out animal testing, regulatory progress for SillaJen’s oncology pipeline BAL0891, and the acquisition of all related patents and rights from the original developer.
On April 21, the company announced that the FDA had approved an amendment to its Phase 1 IND application for BAL0891, expanding the trial to include patients with acute myeloid leukemia (AML). The trial is expected to conclude by March 24, 2025.
A day later, SillaJen revealed it acquired global rights and patents for BAL0891 from Dutch biotech firm Crossfire for 2 million Swiss francs (approximately 3.5 billion won). The amended deal also waived future obligations for milestone and royalty payments, which could have totaled up to CHF 172 million (approx. 300.5 billion won).
SillaJen is also viewed as a likely beneficiary of the FDA’s animal testing reform, as it is the first Korean company to adopt organoid-based models in anticancer drug development. PharmEdaily covered the company’s progress in a report titled “Prepared SillaJen: Korea’s First Organoid-Driven Drug Developer.”
GLPharmTech. Surges on Phase 3 Success in Dry Eye Drug
GLPharmTech. shares soared 29.95% to close at 1,419 won after the company announced that its Phase 3 trial for a dry eye disease treatment, GLH8NDE, showed both efficacy and safety. The drug is co-developed with Aju Pharm.
GLH8NDE contains the novel compound recorflavone and was originally licensed in from Dong-A ST in 2017. According to Phase 3 results, the treatment showed statistically significant improvement in corneal staining scores at week 12?the trial’s primary endpoint. The change in total corneal staining score (TCSS) from baseline at week 12 was -3.00 ± 0.13 in the test group versus -2.63 ± 0.13 in the placebo group, with a statistically significant difference of -0.36 (P=0.0490, ANCOVA).
Secondary endpoints also showed positive outcomes, including conjunctival staining, ocular discomfort scores (ODS), and Ocular Surface Disease Index (OSDI). No significant difference in adverse events was observed between groups, confirming the drug’s safety.
The company plans to file for marketing approval with Korea’s Ministry of Food and Drug Safety (MFDS) in the second half of 2025 and is targeting product launch by 2026 following price negotiations. GLPharmTech. also holds global rights to GLH8NDE and is considering potential out-licensing deals.
A spokesperson said, “The results confirm excellent efficacy with minimal side effects. We’re in active discussions with overseas partners in markets including the U.S., China, and Japan.”
Inventage Lab Stock Recovers Dramatically on Oral Obesity Drug Data
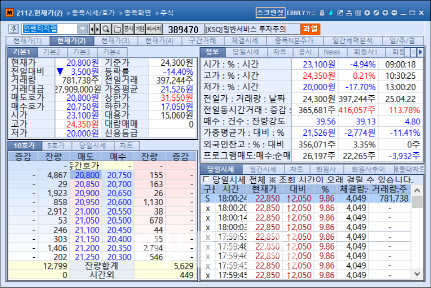
Inventage Lab, Inc. experienced a dramatic reversal on 23 April. After closing down 14.4% at 20,800 won during the regular session, the stock surged 9.86% in after-hours trading to hit the daily upper limit. The rebound followed news that the company’s once-weekly oral obesity treatment showed superior drug absorption compared to major global competitors.
According to the company, the treatment demonstrated not only stable pharmacokinetics (PK) but also a drug absorption rate exceeding 24%. By comparison, Novo Nordisk’s FDA-approved oral obesity drug, Rybelsus, has an absorption rate of just 0.5~1%.
Oral obesity treatments have long struggled with poor absorption rates, prompting most products to be delivered via injection. Inventage’s candidate may overcome this barrier, positioning it as a viable alternative to injectable obesity therapies.
The company used its proprietary IVL-PePOFluidic platform to develop the oral formulation. IVL-PePOFluidic is a platform that converts peptide-based drugs into oral formulations. Inventage Lab, Inc. is already known for its IVL-DrugFluidic, a platform for long-acting injectable formulations.
“Our oral obesity treatment is being developed using our second platform, IVL-PePOFluidic,” said a company official. “The company obtained very promising data by applying the platform to semaglutide, and now plans to expand its application to other drug candidates as well.”
|
According to KG Zeroin’s MP Doctor platform (formerly Market Point), shares of SillaJen surged 950 won, or 29.97%, to close at 4,120 won. This followed a 29.92% jump the previous session, marking two consecutive limit-up days. The stock has now risen for ten straight trading sessions.
The rally was driven by several positive developments: anticipated benefits from the FDA’s plan to phase out animal testing, regulatory progress for SillaJen’s oncology pipeline BAL0891, and the acquisition of all related patents and rights from the original developer.
On April 21, the company announced that the FDA had approved an amendment to its Phase 1 IND application for BAL0891, expanding the trial to include patients with acute myeloid leukemia (AML). The trial is expected to conclude by March 24, 2025.
A day later, SillaJen revealed it acquired global rights and patents for BAL0891 from Dutch biotech firm Crossfire for 2 million Swiss francs (approximately 3.5 billion won). The amended deal also waived future obligations for milestone and royalty payments, which could have totaled up to CHF 172 million (approx. 300.5 billion won).
SillaJen is also viewed as a likely beneficiary of the FDA’s animal testing reform, as it is the first Korean company to adopt organoid-based models in anticancer drug development. PharmEdaily covered the company’s progress in a report titled “Prepared SillaJen: Korea’s First Organoid-Driven Drug Developer.”
GLPharmTech. Surges on Phase 3 Success in Dry Eye Drug
GLPharmTech. shares soared 29.95% to close at 1,419 won after the company announced that its Phase 3 trial for a dry eye disease treatment, GLH8NDE, showed both efficacy and safety. The drug is co-developed with Aju Pharm.
GLH8NDE contains the novel compound recorflavone and was originally licensed in from Dong-A ST in 2017. According to Phase 3 results, the treatment showed statistically significant improvement in corneal staining scores at week 12?the trial’s primary endpoint. The change in total corneal staining score (TCSS) from baseline at week 12 was -3.00 ± 0.13 in the test group versus -2.63 ± 0.13 in the placebo group, with a statistically significant difference of -0.36 (P=0.0490, ANCOVA).
Secondary endpoints also showed positive outcomes, including conjunctival staining, ocular discomfort scores (ODS), and Ocular Surface Disease Index (OSDI). No significant difference in adverse events was observed between groups, confirming the drug’s safety.
The company plans to file for marketing approval with Korea’s Ministry of Food and Drug Safety (MFDS) in the second half of 2025 and is targeting product launch by 2026 following price negotiations. GLPharmTech. also holds global rights to GLH8NDE and is considering potential out-licensing deals.
A spokesperson said, “The results confirm excellent efficacy with minimal side effects. We’re in active discussions with overseas partners in markets including the U.S., China, and Japan.”
Inventage Lab Stock Recovers Dramatically on Oral Obesity Drug Data
Inventage Lab, Inc. experienced a dramatic reversal on 23 April. After closing down 14.4% at 20,800 won during the regular session, the stock surged 9.86% in after-hours trading to hit the daily upper limit. The rebound followed news that the company’s once-weekly oral obesity treatment showed superior drug absorption compared to major global competitors.
|
Oral obesity treatments have long struggled with poor absorption rates, prompting most products to be delivered via injection. Inventage’s candidate may overcome this barrier, positioning it as a viable alternative to injectable obesity therapies.
The company used its proprietary IVL-PePOFluidic platform to develop the oral formulation. IVL-PePOFluidic is a platform that converts peptide-based drugs into oral formulations. Inventage Lab, Inc. is already known for its IVL-DrugFluidic, a platform for long-acting injectable formulations.
“Our oral obesity treatment is being developed using our second platform, IVL-PePOFluidic,” said a company official. “The company obtained very promising data by applying the platform to semaglutide, and now plans to expand its application to other drug candidates as well.”
김새미 bird@