Biotech Rallies as Four Firms Join Korea’s Top 10 Stock Gainers[K-Bio Pulse]
created on 05/28/2025 7:48:35 AM
Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
This article was released as Pharm Edaily Premium Content on 05/28/2025 7:48:35 AM
Subscribe
[Yu Jin-hee, Edaily Reporter] SEOUL, South Korea-Biopharmaceutical and medical device firms showed strong momentum in the South Korean stock market on May 27, with four of the day’s top 10 gainers coming from the bio sector. The surge appears to reflect a combination of favorable market conditions and tangible progress at individual companies.
Cellid Soars 147% in 7 Days; ABL Bio Hits 52-Week High
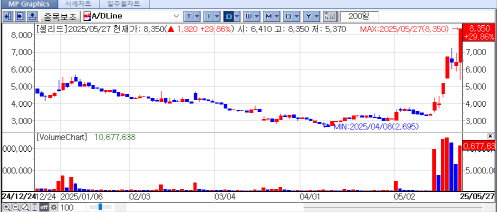
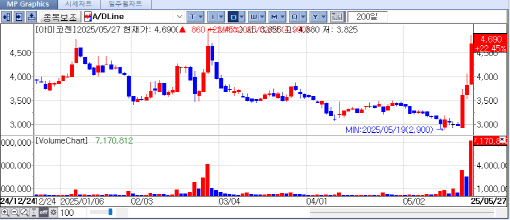
According to data from KG Zeroin’s MP Doctor (formerly MarketPoint), shares of Cellid, ABL Bio, Amicogen, and FineMediX climbed sharply by the close, rising 29.86% (₩8,350), 22.55% (₩82,600), 22.45% (₩4,690), and 20.16% (₩1,560), respectively.
Cellid led the rally with a staggering 147.04% increase over the past seven trading days, up from ₩3,380 on May 16. While some of the momentum may be linked to the resurgence of COVID-19 and investor interest in related themes, the company also announced meaningful developments.
On Tuesday, Cellid revealed that it had completed development and entered production of a new vaccine based on the LP.8.1 COVID-19 variant. The World Health Organization and the Korea Disease Control and Prevention Agency have identified LP.8.1 as a currently spreading strain, and WHO and the European Medicines Agency are considering it as a candidate for the next recommended vaccine antigen. Cellid plans to pursue expedited regulatory approval based on its ongoing Phase 3 global trials of an Omicron-targeted vaccine.
“We are fully prepared for commercialization of a variant-responsive COVID-19 vaccine,” said Cellid CEO Chang-yul Kang. “We will continue leveraging our proprietary vaccine library to address emerging mutations.”
Still, Cellid’s heavy reliance on COVID-related products raises caution among investors. The Korea Exchange (KRX) announced that Cellid will be designated as an investment warning stock on May 28 due to its recent sharp price increases.
“Cellid’s stock has surged rapidly and may be subject to a trading halt,” said the exchange, urging investors to exercise caution.
An industry insider noted, “Experts largely agree that the COVID resurgence is unlikely to last long. Chasing theme stocks blindly could lead to capital being tied up for extended periods.”
ABL Bio drew investor attention by setting a new 52-week high, buoyed by a major licensing deal announced last month. The company signed an agreement with GlaxoSmithKline (GSK) to license out its blood-brain barrier (BBB) penetration platform, Grabody-B, in a deal worth £2.14 billion (approximately ₩4.1 trillion).
Excluding reversals, this is the second-largest license-out deal in Korea’s biotech history, following a 2020 agreement between Alteogen and MSD. ABL Bio has already received ₩73.9 billion in upfront payments from GSK and has achieved cumulative licensing deal value nearing ₩7 trillion.
“We are committed to addressing unmet medical needs,” an ABL Bio spokesperson said. “If we succeed in helping patients who suffer, we believe our corporate value will naturally rise.”
FineMediX Gains on FDA Clearance; Amicogen Validated by Global Journal
FineMediX shares surged following the FDA’s approval of its hemostatic endoscopic device, Clear Hemoglasper, which uses high-frequency current to cauterize exposed vessels during gastrointestinal bleeding. It is the first domestically developed product of its kind.
The company has already been exporting the product to Asia and Europe and now aims to expand into Japan and the Americas. FineMediX plans to build global distribution channels across all 11 of its FDA-cleared products. At this year’s Digestive Disease Week in San Diego, FineMediX held discussions with UK-based Creo Medical about U.S. market testing.
“With the U.S. accounting for 40% of the global endoscopic device market, this approval validates our product’s excellence and positions us to accelerate U.S. expansion,” a company representative said.
Amicogen, meanwhile, drew attention after peer-reviewed publication of research validating its proprietary Low Molecular Collagen Peptide AG. The study, published in Nutrition Research and Practice, demonstrated that the ingredient significantly improved antioxidant biomarkers (SOD and GSH) in aged mice over 12 weeks, showing potential beyond skin health, including for metabolic support in aging.
“This publication confirms that our collagen peptide can play a broader role in aging-related health,” said an Amicogen spokesperson. “We will continue innovating to strengthen our global competitiveness in the collagen market.”
|
Cellid Soars 147% in 7 Days; ABL Bio Hits 52-Week High
According to data from KG Zeroin’s MP Doctor (formerly MarketPoint), shares of Cellid, ABL Bio, Amicogen, and FineMediX climbed sharply by the close, rising 29.86% (₩8,350), 22.55% (₩82,600), 22.45% (₩4,690), and 20.16% (₩1,560), respectively.
Cellid led the rally with a staggering 147.04% increase over the past seven trading days, up from ₩3,380 on May 16. While some of the momentum may be linked to the resurgence of COVID-19 and investor interest in related themes, the company also announced meaningful developments.
On Tuesday, Cellid revealed that it had completed development and entered production of a new vaccine based on the LP.8.1 COVID-19 variant. The World Health Organization and the Korea Disease Control and Prevention Agency have identified LP.8.1 as a currently spreading strain, and WHO and the European Medicines Agency are considering it as a candidate for the next recommended vaccine antigen. Cellid plans to pursue expedited regulatory approval based on its ongoing Phase 3 global trials of an Omicron-targeted vaccine.
“We are fully prepared for commercialization of a variant-responsive COVID-19 vaccine,” said Cellid CEO Chang-yul Kang. “We will continue leveraging our proprietary vaccine library to address emerging mutations.”
Still, Cellid’s heavy reliance on COVID-related products raises caution among investors. The Korea Exchange (KRX) announced that Cellid will be designated as an investment warning stock on May 28 due to its recent sharp price increases.
“Cellid’s stock has surged rapidly and may be subject to a trading halt,” said the exchange, urging investors to exercise caution.
An industry insider noted, “Experts largely agree that the COVID resurgence is unlikely to last long. Chasing theme stocks blindly could lead to capital being tied up for extended periods.”
ABL Bio drew investor attention by setting a new 52-week high, buoyed by a major licensing deal announced last month. The company signed an agreement with GlaxoSmithKline (GSK) to license out its blood-brain barrier (BBB) penetration platform, Grabody-B, in a deal worth £2.14 billion (approximately ₩4.1 trillion).
Excluding reversals, this is the second-largest license-out deal in Korea’s biotech history, following a 2020 agreement between Alteogen and MSD. ABL Bio has already received ₩73.9 billion in upfront payments from GSK and has achieved cumulative licensing deal value nearing ₩7 trillion.
“We are committed to addressing unmet medical needs,” an ABL Bio spokesperson said. “If we succeed in helping patients who suffer, we believe our corporate value will naturally rise.”
|
FineMediX Gains on FDA Clearance; Amicogen Validated by Global Journal
FineMediX shares surged following the FDA’s approval of its hemostatic endoscopic device, Clear Hemoglasper, which uses high-frequency current to cauterize exposed vessels during gastrointestinal bleeding. It is the first domestically developed product of its kind.
The company has already been exporting the product to Asia and Europe and now aims to expand into Japan and the Americas. FineMediX plans to build global distribution channels across all 11 of its FDA-cleared products. At this year’s Digestive Disease Week in San Diego, FineMediX held discussions with UK-based Creo Medical about U.S. market testing.
“With the U.S. accounting for 40% of the global endoscopic device market, this approval validates our product’s excellence and positions us to accelerate U.S. expansion,” a company representative said.
Amicogen, meanwhile, drew attention after peer-reviewed publication of research validating its proprietary Low Molecular Collagen Peptide AG. The study, published in Nutrition Research and Practice, demonstrated that the ingredient significantly improved antioxidant biomarkers (SOD and GSH) in aged mice over 12 weeks, showing potential beyond skin health, including for metabolic support in aging.
“This publication confirms that our collagen peptide can play a broader role in aging-related health,” said an Amicogen spokesperson. “We will continue innovating to strengthen our global competitiveness in the collagen market.”
유진희 sadend@







![[바이오맥짚기]'액체생검' 온코크로스, 주가 급등...'오너리스크' 신풍제약은 급락](https://image.edaily.co.kr/images/vision/files/NP/S/2025/05/PS25052900298b.jpg)
