Precision Bio Surges on FDA Approval Kolon, GI Innovation Also Gain[K-bio pulse]
created on 06/20/2025 7:49:07 AM
Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
This article was released as Pharm Edaily Premium Content on 06/20/2025 7:49:07 AM
Subscribe
[Song young-doo, Edaily Reporter] On June 19, while most Korean pharmaceutical and biotech stocks weakened, shares of Precision Bio hit the upper trading limit after its U.S. subsidiary received full FDA approval for a combo diagnostic kit that detects both COVID-19 and influenza. Kolon TissueGene and GI Innovation, which are participating in the global Bio USA convention, also saw significant stock gains driven by expectations surrounding their respective milestones.
Precision Bio Soars on FDA Clearance of Combo Test Kit
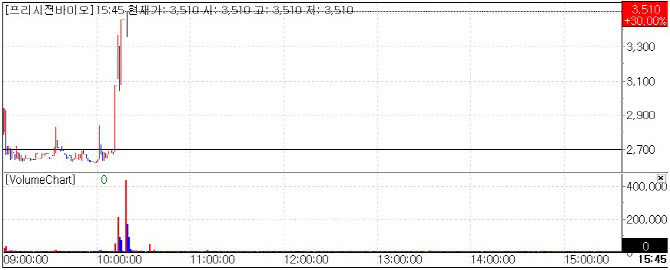
According to KG Zeroin’s MP DOCTOR(formerly Market Point), Precision Bio closed at KRW 3,510, up 30%(KRW 810) from the previous session.
Although the stock opened weak, it surged sharply after 10 a.m. following a press release announcing FDA 510(k) clearance for Nano-Check™ Influenza+COVID-19 Dual Test, developed by its U.S. subsidiary Nanoditech. The point-of-care(POC) kit can simultaneously detect COVID-19 and Influenza A/B and is designed for professional use in hospitals, pharmacies, and public health centers.
This marks the first full FDA clearance for a combo diagnostic kit for professionals, following a period in which only Emergency Use Authorizations were granted during the pandemic. A Precision Bio spokesperson explained, “The stock surge is attributed to the removal of uncertainty surrounding full approval in the post-pandemic era.”
With this clearance, Precision Bio now offers a comprehensive respiratory diagnostic portfolio, including previously approved COVID-19 and RSV kits. The company stated that the RSV kit, after securing a U.S. supply deal with Sekisui in March, received Korean MFDS import clearance in May, enabling domestic sales.
The company also noted rising global demand for combo kits amid recurring COVID-19 outbreaks. “There is sustained demand in the U.S. for combo kits,” the company said, emphasizing that a robust portfolio is essential for partnerships with global distributors, some of whom are already in contract or negotiation.
Kolon TissueGene Rises on InvoSA Expectations and Bio USA Buzz
Kolon TissueGene, also at Bio USA, saw its stock surge in the afternoon after CEO Jeon Seung-ho remarked in a media interview about strong global interest in Invossa, its investigational osteoarthritis cell therapy.
The stock climbed 20.68%(KRW 7,900) to close at KRW 46,100. Since opening the year at KRW 24,200 on January 2, shares have risen 90.5% over six months on continued hopes for Invossa‘s commercialization.
Invossa aims to become the world’s first DMOAD(disease-modifying osteoarthritis drug) that structurally halts disease progression. CEO Jeon stated that over 200 companies at Bio USA requested meetings, and the company narrowed it down to 35 for meaningful partnership discussions.
Kolon TissueGene has completed dosing in its U.S. Phase 3 trial involving 1,020 patients and plans to file for FDA approval in Q1 2027. The osteoarthritis drug market across the G7 countries was estimated at KRW 3 trillion in 2024 and is projected to reach KRW 5.5 trillion by 2031 with a 5.3% CAGR. The global market is expected to exceed KRW 12 trillion.
GI Innovation Gains on U.S. Patent and Upcoming Conference Call
GI Innovation also saw a sharp midday rally, closing up 7.78%(KRW 1,700) at KRW 23,550. The stock had been rising gradually before surging after noon.
The company announced in a press release that it had received a U.S. material patent for GI-108, a fourth-generation metabolic immuno-oncology drug. The patent secures exclusive rights to the drug’s unique bispecific fusion protein structure and mechanism of action, bolstering its intellectual property ahead of potential global licensing and commercialization.
Currently meeting with multiple partners at Bio USA, GI Innovation expects the patent to strengthen its negotiation leverage and investor appeal. GI-108 is undergoing Phase 1/2a trials in patients with NSCLC, pancreatic cancer, and renal cell carcinoma, with clinical sites including Yonsei Severance Hospital, Samsung Medical Center, and Asan Medical Center.
Following its presentation at the JPMorgan Healthcare Conference, the company began actively pursuing out-licensing deals for GI-108, which, alongside GI-101A and GI-102, comprises its core pipeline.
Importantly, GI Innovation plans to hold a conference call on July 3, where it will disclose early-phase clinical results of GI-102 in combination with Keytruda.
A company representative commented, “Today’s rally was driven by multiple factors, including the U.S. patent news and anticipation surrounding the upcoming conference call.”
|
Precision Bio Soars on FDA Clearance of Combo Test Kit
According to KG Zeroin’s MP DOCTOR(formerly Market Point), Precision Bio closed at KRW 3,510, up 30%(KRW 810) from the previous session.
Although the stock opened weak, it surged sharply after 10 a.m. following a press release announcing FDA 510(k) clearance for Nano-Check™ Influenza+COVID-19 Dual Test, developed by its U.S. subsidiary Nanoditech. The point-of-care(POC) kit can simultaneously detect COVID-19 and Influenza A/B and is designed for professional use in hospitals, pharmacies, and public health centers.
This marks the first full FDA clearance for a combo diagnostic kit for professionals, following a period in which only Emergency Use Authorizations were granted during the pandemic. A Precision Bio spokesperson explained, “The stock surge is attributed to the removal of uncertainty surrounding full approval in the post-pandemic era.”
With this clearance, Precision Bio now offers a comprehensive respiratory diagnostic portfolio, including previously approved COVID-19 and RSV kits. The company stated that the RSV kit, after securing a U.S. supply deal with Sekisui in March, received Korean MFDS import clearance in May, enabling domestic sales.
The company also noted rising global demand for combo kits amid recurring COVID-19 outbreaks. “There is sustained demand in the U.S. for combo kits,” the company said, emphasizing that a robust portfolio is essential for partnerships with global distributors, some of whom are already in contract or negotiation.
Kolon TissueGene Rises on InvoSA Expectations and Bio USA Buzz
Kolon TissueGene, also at Bio USA, saw its stock surge in the afternoon after CEO Jeon Seung-ho remarked in a media interview about strong global interest in Invossa, its investigational osteoarthritis cell therapy.
The stock climbed 20.68%(KRW 7,900) to close at KRW 46,100. Since opening the year at KRW 24,200 on January 2, shares have risen 90.5% over six months on continued hopes for Invossa‘s commercialization.
Invossa aims to become the world’s first DMOAD(disease-modifying osteoarthritis drug) that structurally halts disease progression. CEO Jeon stated that over 200 companies at Bio USA requested meetings, and the company narrowed it down to 35 for meaningful partnership discussions.
Kolon TissueGene has completed dosing in its U.S. Phase 3 trial involving 1,020 patients and plans to file for FDA approval in Q1 2027. The osteoarthritis drug market across the G7 countries was estimated at KRW 3 trillion in 2024 and is projected to reach KRW 5.5 trillion by 2031 with a 5.3% CAGR. The global market is expected to exceed KRW 12 trillion.
GI Innovation Gains on U.S. Patent and Upcoming Conference Call
GI Innovation also saw a sharp midday rally, closing up 7.78%(KRW 1,700) at KRW 23,550. The stock had been rising gradually before surging after noon.
The company announced in a press release that it had received a U.S. material patent for GI-108, a fourth-generation metabolic immuno-oncology drug. The patent secures exclusive rights to the drug’s unique bispecific fusion protein structure and mechanism of action, bolstering its intellectual property ahead of potential global licensing and commercialization.
Currently meeting with multiple partners at Bio USA, GI Innovation expects the patent to strengthen its negotiation leverage and investor appeal. GI-108 is undergoing Phase 1/2a trials in patients with NSCLC, pancreatic cancer, and renal cell carcinoma, with clinical sites including Yonsei Severance Hospital, Samsung Medical Center, and Asan Medical Center.
Following its presentation at the JPMorgan Healthcare Conference, the company began actively pursuing out-licensing deals for GI-108, which, alongside GI-101A and GI-102, comprises its core pipeline.
Importantly, GI Innovation plans to hold a conference call on July 3, where it will disclose early-phase clinical results of GI-102 in combination with Keytruda.
A company representative commented, “Today’s rally was driven by multiple factors, including the U.S. patent news and anticipation surrounding the upcoming conference call.”
송영두 songzio@






![신풍제약, ‘피라맥스’ 유럽 특허로 이틀째 上…지니너스·비올도 강세[바이오 맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/06/PS25061900344b.jpg)