P QuantaMatrix Boosts Market Hopes with U.S.-Backed CARB-X Project[K-Bio Pulse]
created on 09/04/2025 8:14:58 AM
Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
This article was released as Pharm Edaily Premium Content on 09/04/2025 8:14:58 AM
Subscribe
[Yu Jin-hee, Edaily Reporter] SEOUL, South Korea-On Sept. 3, South Korea’s stock market saw a notable surge in biopharma and medical device shares, with five companies landing in the day’s top 20 gainers: IMBdx, HEM Pharma, GI Innovation, SCM Lifescience, and QuantaMatrix.
QuantaMatrix Sees Strong Upside Potential
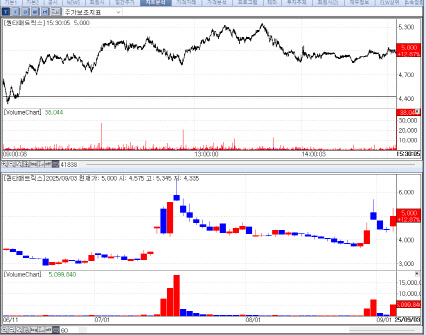
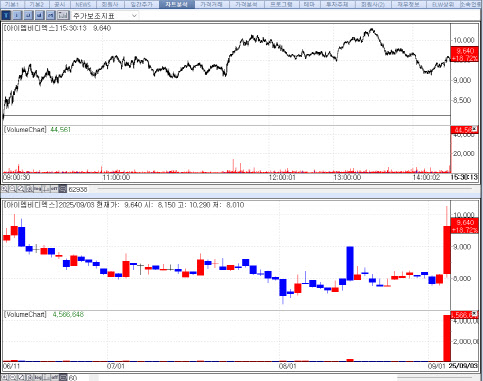
According to KG Zeroin’s MP Doctor platform (formerly Market Point), shares of IMBdx, HEM Pharma, GI Innovation, SCM Lifescience, and QuantaMatrix climbed 18.72% (closing at 9,640 won), 16.55% (22,750 won), 14.85% (18,860 won), 14.30% (1,111 won), and 12.87% (5,000 won), respectively, compared with the previous day.
QuantaMatrix at one point surged more than 20% during trading, drawing strong investor attention. The rally was partly attributed to a premium PharmEdaily pay-to-read article titled “QuantaMatrix Breaks Into CARB-X, Following $420M M&A Precedent”, which was later made publicly available on portal sites in the afternoon.
The report highlighted that QuantaMatrix, a microbial diagnostics developer, was recently selected for support by CARB-X, a U.S.-led global antimicrobial resistance initiative. It noted that a previous CARB-X-backed biotech venture was acquired by the world’s top diagnostics firm in a 540 billion won ($420 million) deal, raising expectations for QuantaMatrix’s future valuation.
CARB-X is a global infectious disease fund spearheaded by the U.S. Biomedical Advanced Research and Development Authority (BARDA), with participation from the U.K. Department of Health, Health Canada, the Bill & Melinda Gates Foundation, and Wellcome Trust. Since its launch in 2017, it has funded more than 100 projects, some of which have been commercialized. The program’s acceptance rate is highly competitive, with odds typically worse than 1 in 20.
This year, QuantaMatrix became the first Korean company chosen by CARB-X, securing funding for its neonatal sepsis diagnostics platform. The project aims to develop technology capable of detecting more than 50 pathogens from just 1 milliliter of neonatal blood within six hours—a task considered nearly impossible given that similar tests in adults often take more than a full day.
Industry analysts estimate the global sepsis diagnostics market at roughly 25 trillion won, spanning three main segments: blood culture (7 trillion won), microbial identification (4 trillion won), and antimicrobial susceptibility testing (4 trillion won). QuantaMatrix’s solution, branded as “uRAST,” seeks to integrate all three into a single platform, with commercialization targeted within three years.
“We demonstrated the feasibility of neonatal sepsis diagnostics using our proprietary technology in a Nature publication last year,” CEO Kwon Sung-hoon said. “By extending this platform to both adult and neonatal cases, we aim to strengthen our foothold in the clinical microbiology testing market.”
IMBdx and GI Innovation Also Impress
Other standout performers included IMBdx, which launched an upgraded version of its liquid biopsy platform, AlphaLiquid 3.0 (CancerDx200). The test expands its genomic coverage from 118 to 192 genes, incorporating all FDA-approved biomarkers as well as key genetic markers relevant for solid tumors such as prostate, lung, and pancreatic cancers.
IMBdx plans to roll out a prostate and breast cancer-focused liquid biopsy test by year-end, along with a large-scale research-use panel, AlphaLiquid 1000. The company is also preparing to enter the tissue-based cancer genomics market, strengthening its leadership in precision medicine.
“This upgrade nearly doubles the genomic coverage while retaining reimbursement eligibility, delivering a tangible benefit to both patients and clinicians,” said Cha Yong-jun, chief medical officer. “We aim to boost treatment accessibility for Korean cancer patients and lead the global precision medicine paradigm.”
GI Innovation also fueled optimism after announcing that its next-generation cytokine immunotherapy, GI-102, achieved a complete response in a patient during a Phase 1 monotherapy trial with subcutaneous administration. The company is also testing GI-101 and GI-102 in combination with Merck’s immunotherapy Keytruda (pembrolizumab).
“Four patients who had previously failed to respond to immunotherapy all showed tumor shrinkage of more than 30% in a Phase 2 study combining GI-102 with Keytruda,” said Executive Vice President Yoon Nari. “Based on these results, we aim to pursue FDA accelerated approval by the third quarter of 2028.”
Meanwhile, microbiome developer HEM Pharma and cell therapy company SCM Lifescience also saw double-digit share gains despite no major announcements.
|
QuantaMatrix Sees Strong Upside Potential
According to KG Zeroin’s MP Doctor platform (formerly Market Point), shares of IMBdx, HEM Pharma, GI Innovation, SCM Lifescience, and QuantaMatrix climbed 18.72% (closing at 9,640 won), 16.55% (22,750 won), 14.85% (18,860 won), 14.30% (1,111 won), and 12.87% (5,000 won), respectively, compared with the previous day.
QuantaMatrix at one point surged more than 20% during trading, drawing strong investor attention. The rally was partly attributed to a premium PharmEdaily pay-to-read article titled “QuantaMatrix Breaks Into CARB-X, Following $420M M&A Precedent”, which was later made publicly available on portal sites in the afternoon.
The report highlighted that QuantaMatrix, a microbial diagnostics developer, was recently selected for support by CARB-X, a U.S.-led global antimicrobial resistance initiative. It noted that a previous CARB-X-backed biotech venture was acquired by the world’s top diagnostics firm in a 540 billion won ($420 million) deal, raising expectations for QuantaMatrix’s future valuation.
CARB-X is a global infectious disease fund spearheaded by the U.S. Biomedical Advanced Research and Development Authority (BARDA), with participation from the U.K. Department of Health, Health Canada, the Bill & Melinda Gates Foundation, and Wellcome Trust. Since its launch in 2017, it has funded more than 100 projects, some of which have been commercialized. The program’s acceptance rate is highly competitive, with odds typically worse than 1 in 20.
This year, QuantaMatrix became the first Korean company chosen by CARB-X, securing funding for its neonatal sepsis diagnostics platform. The project aims to develop technology capable of detecting more than 50 pathogens from just 1 milliliter of neonatal blood within six hours—a task considered nearly impossible given that similar tests in adults often take more than a full day.
Industry analysts estimate the global sepsis diagnostics market at roughly 25 trillion won, spanning three main segments: blood culture (7 trillion won), microbial identification (4 trillion won), and antimicrobial susceptibility testing (4 trillion won). QuantaMatrix’s solution, branded as “uRAST,” seeks to integrate all three into a single platform, with commercialization targeted within three years.
“We demonstrated the feasibility of neonatal sepsis diagnostics using our proprietary technology in a Nature publication last year,” CEO Kwon Sung-hoon said. “By extending this platform to both adult and neonatal cases, we aim to strengthen our foothold in the clinical microbiology testing market.”
|
IMBdx and GI Innovation Also Impress
Other standout performers included IMBdx, which launched an upgraded version of its liquid biopsy platform, AlphaLiquid 3.0 (CancerDx200). The test expands its genomic coverage from 118 to 192 genes, incorporating all FDA-approved biomarkers as well as key genetic markers relevant for solid tumors such as prostate, lung, and pancreatic cancers.
IMBdx plans to roll out a prostate and breast cancer-focused liquid biopsy test by year-end, along with a large-scale research-use panel, AlphaLiquid 1000. The company is also preparing to enter the tissue-based cancer genomics market, strengthening its leadership in precision medicine.
“This upgrade nearly doubles the genomic coverage while retaining reimbursement eligibility, delivering a tangible benefit to both patients and clinicians,” said Cha Yong-jun, chief medical officer. “We aim to boost treatment accessibility for Korean cancer patients and lead the global precision medicine paradigm.”
GI Innovation also fueled optimism after announcing that its next-generation cytokine immunotherapy, GI-102, achieved a complete response in a patient during a Phase 1 monotherapy trial with subcutaneous administration. The company is also testing GI-101 and GI-102 in combination with Merck’s immunotherapy Keytruda (pembrolizumab).
“Four patients who had previously failed to respond to immunotherapy all showed tumor shrinkage of more than 30% in a Phase 2 study combining GI-102 with Keytruda,” said Executive Vice President Yoon Nari. “Based on these results, we aim to pursue FDA accelerated approval by the third quarter of 2028.”
Meanwhile, microbiome developer HEM Pharma and cell therapy company SCM Lifescience also saw double-digit share gains despite no major announcements.
유진희 sadend@



![i-Sens Soars 23% on EU CGM Expansion [K-bio pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26021300327b.jpg)


![냉탕 온탕 오간 에이프릴바이오…실적 호조에 로킷·휴젤 상승[바이오맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26021200275b.jpg)
