G2G Bio Surges; Gates’ Visit Boosts Vaccine Stocks[K-bio pulse]
created on 08/19/2025 9:20:59 AM
Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
This article was released as Pharm Edaily Premium Content on 08/19/2025 9:20:59 AM
Subscribe
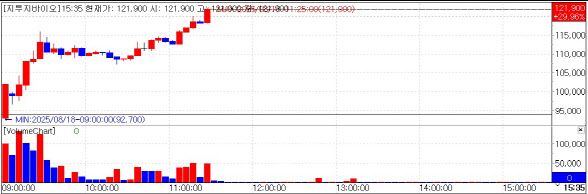
[Kim Jinsoo, Edaily Reporter] Shares of G2G Bio soared to the daily upper limit on Monday, extending gains for a second straight session, as anticipation grew over Bill Gates’ upcoming visit to South Korea to discuss vaccine cooperation with domestic biopharma firms.
G2G Bio Continues Uptrend
On Aug. 18, G2G Bio closed at 121,000 won, up by the daily maximum, according to KG Zeroin MP Doctor (formerly MarketPoint). The stock has surged since its Aug. 14 debut, when it ended 61.7 percent above its IPO price of 58,000 won.
The company announced on Aug. 12 that it was selected for the Ministry of Trade, Industry and Energy’s 2025 material and component technology development program. The two-and-a-half-year project will provide about 2.5 billion won ($25 million) in funding. G2G Bio plans to develop a once-monthly immunosuppressant for transplant patients, using its proprietary drug delivery platform, InnoLAMP. The microsphere-based technology slowly releases drugs into the body, reducing the burden of daily oral dosing and stabilizing blood concentrations, a critical factor in transplant safety.
A company official said preclinical tests have already shown that high-dose formulations can be controlled within the body, and the firm is targeting global markets including the U.S. and Europe with plans for licensing deals.
Gates’ Visit Lifts Vaccine Makers
Shares of vaccine developers also jumped after reports that Microsoft co-founder and Gates Foundation Co-Chair Bill Gates will visit Korea on Aug. 21 to meet with pharmaceutical and biotech companies.
Eubiologics climbed as much as 14.4 percent intraday before closing up 3.7 percent at 13,180 won. SK Bioscience also gained 3.1 percent, finishing at 48,100 won after briefly topping 50,000 won.
The Gates Foundation has supported both companies in the past. In 2023, it funded Eubiologics’ global Phase 3 trial of its five-valent meningococcal conjugate vaccine (EuMCV5). In 2022, the foundation provided $4.2 million to help expand the company’s cholera vaccine production.
SK Bioscience’s partnership with the Gates Foundation dates back to 2013. In 2016, the foundation contributed about $1.42 million to support pneumococcal vaccine development for low-income countries. During the COVID-19 pandemic, SK Bioscience received $3.6 million in funding, followed by expanded collaboration with the Coalition for Epidemic Preparedness Innovations (CEPI) on the GBP510 next-generation COVID-19 vaccine. The company later developed the typhoid conjugate vaccine SKYTyphoid and Korea’s first COVID-19 vaccine, SKYCovione, both backed by the Gates Foundation.
Executives from SK Group, SK Bioscience, and the Gates Foundation met in Seoul and Seattle in 2022 to explore ways to expand cooperation and strengthen pandemic preparedness.
Observers expect Gates’ upcoming visit to focus on vaccine distribution for low-income nations and new joint projects.
SK Bioscience declined to comment on share-price movements.
DXVX Jumps on $500M U.S. Licensing Deal
DXVX shares also rallied, closing up 12.6 percent at 2,590 won after rising as much as 25.6 percent intraday. The surge followed news that its subsidiary Evixgen signed a 500 billion won ($500 million) licensing agreement with a U.S. biotech firm for its advanced drug delivery platform, ACP.
Under the deal, Evixgen will receive milestone payments and royalties while granting limited exclusive rights to apply the ACP technology to new drug candidates. The peptide-based platform has demonstrated the ability to cross the blood-brain barrier in animal studies, a major hurdle in central nervous system drug development.
DXVX, which holds a 66.2 percent stake in Evixgen, led the negotiations after receiving business development rights from its subsidiary. Officials said the agreement could pave the way for additional licensing deals given the platform’s broad applications.
“This deal not only validates Evixgen’s ACP technology but also enhances DXVX’s business development track record,” a company spokesperson said.
|
On Aug. 18, G2G Bio closed at 121,000 won, up by the daily maximum, according to KG Zeroin MP Doctor (formerly MarketPoint). The stock has surged since its Aug. 14 debut, when it ended 61.7 percent above its IPO price of 58,000 won.
The company announced on Aug. 12 that it was selected for the Ministry of Trade, Industry and Energy’s 2025 material and component technology development program. The two-and-a-half-year project will provide about 2.5 billion won ($25 million) in funding. G2G Bio plans to develop a once-monthly immunosuppressant for transplant patients, using its proprietary drug delivery platform, InnoLAMP. The microsphere-based technology slowly releases drugs into the body, reducing the burden of daily oral dosing and stabilizing blood concentrations, a critical factor in transplant safety.
A company official said preclinical tests have already shown that high-dose formulations can be controlled within the body, and the firm is targeting global markets including the U.S. and Europe with plans for licensing deals.
Gates’ Visit Lifts Vaccine Makers
Shares of vaccine developers also jumped after reports that Microsoft co-founder and Gates Foundation Co-Chair Bill Gates will visit Korea on Aug. 21 to meet with pharmaceutical and biotech companies.
Eubiologics climbed as much as 14.4 percent intraday before closing up 3.7 percent at 13,180 won. SK Bioscience also gained 3.1 percent, finishing at 48,100 won after briefly topping 50,000 won.
The Gates Foundation has supported both companies in the past. In 2023, it funded Eubiologics’ global Phase 3 trial of its five-valent meningococcal conjugate vaccine (EuMCV5). In 2022, the foundation provided $4.2 million to help expand the company’s cholera vaccine production.
SK Bioscience’s partnership with the Gates Foundation dates back to 2013. In 2016, the foundation contributed about $1.42 million to support pneumococcal vaccine development for low-income countries. During the COVID-19 pandemic, SK Bioscience received $3.6 million in funding, followed by expanded collaboration with the Coalition for Epidemic Preparedness Innovations (CEPI) on the GBP510 next-generation COVID-19 vaccine. The company later developed the typhoid conjugate vaccine SKYTyphoid and Korea’s first COVID-19 vaccine, SKYCovione, both backed by the Gates Foundation.
Executives from SK Group, SK Bioscience, and the Gates Foundation met in Seoul and Seattle in 2022 to explore ways to expand cooperation and strengthen pandemic preparedness.
Observers expect Gates’ upcoming visit to focus on vaccine distribution for low-income nations and new joint projects.
SK Bioscience declined to comment on share-price movements.
DXVX Jumps on $500M U.S. Licensing Deal
DXVX shares also rallied, closing up 12.6 percent at 2,590 won after rising as much as 25.6 percent intraday. The surge followed news that its subsidiary Evixgen signed a 500 billion won ($500 million) licensing agreement with a U.S. biotech firm for its advanced drug delivery platform, ACP.
Under the deal, Evixgen will receive milestone payments and royalties while granting limited exclusive rights to apply the ACP technology to new drug candidates. The peptide-based platform has demonstrated the ability to cross the blood-brain barrier in animal studies, a major hurdle in central nervous system drug development.
DXVX, which holds a 66.2 percent stake in Evixgen, led the negotiations after receiving business development rights from its subsidiary. Officials said the agreement could pave the way for additional licensing deals given the platform’s broad applications.
“This deal not only validates Evixgen’s ACP technology but also enhances DXVX’s business development track record,” a company spokesperson said.



![Biotech stocks tumble on rights offerings[K-bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/08/PS25082000380b.jpg)

![South Korean Biotech Sector Surges on Global Pharma Partnerships[K-bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/08/PS25081800379b.jpg)
