Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
This article was released as Pharm Edaily Premium Content on 07/24/2025 9:00:19 AM
Subscribe
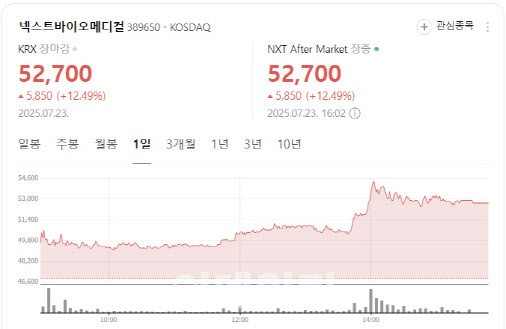
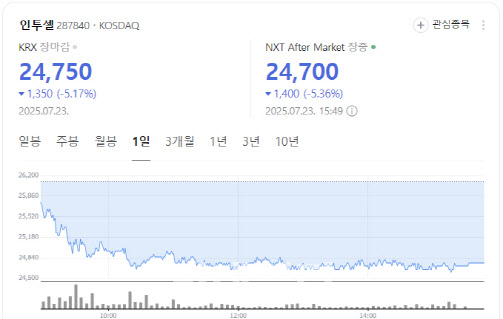
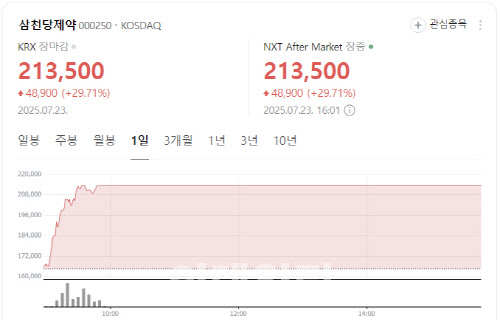
[Seok Ji-hoen, Edaily reporter] Shares of NextBioMedical surged more than 12% Tuesday on growing expectations for the commercialization of its localized pain treatment device, Nexsphere-F. Meanwhile, Intocell fell over 5% as patent risk concerns lingered, and Samchundang Pharm hit the upper limit on news that its oral GLP-1 generic candidate met bioequivalence with the original drug.
“FDA approval and commercialization in sight”
According to MP Doctor (formerly Market Point), NextBioMedical shares rose 12.49% to close at 52,700 won. The rally came after LS Securities released a bullish research report earlier in the day.
In the report titled “Time to Reflect the Value of Nexsphere-F with High Probability of Success,” LS Securities set a new target price of 83,000 won, suggesting a 77.2% upside.
“Nexsphere-F, the world’s first biodegradable embolic agent for pain treatment developed by the company, is preparing to enter clinical trials in the U.S. for FDA approval, bringing it one step closer to commercialization,” said analyst Eun-ae Cho. “Given its differentiated technology, clinical efficacy, and potential for a licensing deal in the U.S., the product has a high probability of global success.”
Nexsphere-F works by temporarily blocking blood flow to abnormal blood vessels responsible for pain, unlike conventional systemic painkillers. The treatment is attracting attention for targeting pathological causes directly and locally.
The company plans to begin U.S. clinical trials in Q3 2025, aiming for FDA approval in 2027. It has already received IDE Category B approval from the Centers for Medicare & Medicaid Services (CMS), allowing Medicare reimbursement during clinical trials, a key advantage for market entry. Nexsphere-F is also designated as a Breakthrough Device and included in the FDA’s TAP (Total Product Life Cycle Advisory Program) initiative.
PharmEdaily previously reported on June 4 that Nexsphere-F had secured both CMS reimbursement support and FDA regulatory guidance, potentially accelerating its path to market in the U.S.
According to industry data, the number of osteoarthritis patients in the U.S. Nexsphere-F’s key target population is expected to grow from 33 million in 2024 to 54 million by 2034. LS Securities forecasts that Nexsphere-F will achieve 100 billion won in revenue in its launch year of 2028 and 42 billion won in operating profit by 2030, with a peak market penetration of 5% by the sixth year. Even under conservative assumptions, its U.S. business value is estimated at 380 billion won.
A company official said, “Nexsphere-F’s value hadn’t been fully reflected in our share price until now, and the market is finally responding.”
Patent risk lingers for Intocell
Shares of Intocell fell 5.17% to 24,750 won, with investors remaining cautious after the recent termination of a licensing deal due to patent risk.
Earlier this month, the company announced that its October 2023 licensing agreement with ABL Bio was canceled. The reason: concerns that its Nexatecan platform may infringe on a Chinese company’s prior patent.
PharmEdaily’s July 18 paywalled article titled “Why Was a Deal Signed Without Patent Registration?” was made freely accessible Tuesday, possibly contributing to the stock‘s decline. The report highlighted that Intocell’s patent application was only in provisional status at the time of the deal and had yet to be formally registered, exposing the company to legal risk.
Although provisional patents confer filing priority, they do not guarantee protection, and the contents are not disclosed for 18 months?making it difficult to assess potential overlap with earlier patents. The incident raised questions about Intocell’s IP readiness and transparency.
While Intocell maintains that pre-registration licensing is standard in the ADC industry, critics argue that both Intocell and ABL Bio underestimated the risks. “Patent registration often takes years, so companies proceed with licensing based on applications,” a biotech VC told Edaily. “Still, more thorough FTO (freedom-to-operate) analysis might have prevented this.”
Samchundang soars on GLP-1 generic breakthrough
Samchundang Pharm shares jumped 29.71% to 213,500 won, hitting the daily upper limit after announcing successful bioequivalence results for its oral GLP-1 generic candidate.
The company said the generic matched the original drug, Novo Nordisk’s Rybelsus, in Cmax and AUC metrics, demonstrating equal absorption.
The market interpreted the results as a breakthrough for the company’s SNAC-free platform technology, S-PASS, which replicates the original formulation’s effect while avoiding patent infringement.
GLP-1 drugs, originally developed for diabetes, are now widely used for obesity treatment and are projected to reach $1.5 trillion in annual global sales. Among them, Rybelsus offers the advantage of oral dosing but has faced supply shortages.
“This is not about improving the original,” a company official said. “It’s about replicating it precisely and getting to market faster than competitors. The results validate the precision absorption control of our S-PASS platform.”
“FDA approval and commercialization in sight”
According to MP Doctor (formerly Market Point), NextBioMedical shares rose 12.49% to close at 52,700 won. The rally came after LS Securities released a bullish research report earlier in the day.
|
“Nexsphere-F, the world’s first biodegradable embolic agent for pain treatment developed by the company, is preparing to enter clinical trials in the U.S. for FDA approval, bringing it one step closer to commercialization,” said analyst Eun-ae Cho. “Given its differentiated technology, clinical efficacy, and potential for a licensing deal in the U.S., the product has a high probability of global success.”
Nexsphere-F works by temporarily blocking blood flow to abnormal blood vessels responsible for pain, unlike conventional systemic painkillers. The treatment is attracting attention for targeting pathological causes directly and locally.
The company plans to begin U.S. clinical trials in Q3 2025, aiming for FDA approval in 2027. It has already received IDE Category B approval from the Centers for Medicare & Medicaid Services (CMS), allowing Medicare reimbursement during clinical trials, a key advantage for market entry. Nexsphere-F is also designated as a Breakthrough Device and included in the FDA’s TAP (Total Product Life Cycle Advisory Program) initiative.
PharmEdaily previously reported on June 4 that Nexsphere-F had secured both CMS reimbursement support and FDA regulatory guidance, potentially accelerating its path to market in the U.S.
According to industry data, the number of osteoarthritis patients in the U.S. Nexsphere-F’s key target population is expected to grow from 33 million in 2024 to 54 million by 2034. LS Securities forecasts that Nexsphere-F will achieve 100 billion won in revenue in its launch year of 2028 and 42 billion won in operating profit by 2030, with a peak market penetration of 5% by the sixth year. Even under conservative assumptions, its U.S. business value is estimated at 380 billion won.
A company official said, “Nexsphere-F’s value hadn’t been fully reflected in our share price until now, and the market is finally responding.”
Patent risk lingers for Intocell
Shares of Intocell fell 5.17% to 24,750 won, with investors remaining cautious after the recent termination of a licensing deal due to patent risk.
|
PharmEdaily’s July 18 paywalled article titled “Why Was a Deal Signed Without Patent Registration?” was made freely accessible Tuesday, possibly contributing to the stock‘s decline. The report highlighted that Intocell’s patent application was only in provisional status at the time of the deal and had yet to be formally registered, exposing the company to legal risk.
Although provisional patents confer filing priority, they do not guarantee protection, and the contents are not disclosed for 18 months?making it difficult to assess potential overlap with earlier patents. The incident raised questions about Intocell’s IP readiness and transparency.
While Intocell maintains that pre-registration licensing is standard in the ADC industry, critics argue that both Intocell and ABL Bio underestimated the risks. “Patent registration often takes years, so companies proceed with licensing based on applications,” a biotech VC told Edaily. “Still, more thorough FTO (freedom-to-operate) analysis might have prevented this.”
Samchundang soars on GLP-1 generic breakthrough
Samchundang Pharm shares jumped 29.71% to 213,500 won, hitting the daily upper limit after announcing successful bioequivalence results for its oral GLP-1 generic candidate.
|
The market interpreted the results as a breakthrough for the company’s SNAC-free platform technology, S-PASS, which replicates the original formulation’s effect while avoiding patent infringement.
GLP-1 drugs, originally developed for diabetes, are now widely used for obesity treatment and are projected to reach $1.5 trillion in annual global sales. Among them, Rybelsus offers the advantage of oral dosing but has faced supply shortages.
“This is not about improving the original,” a company official said. “It’s about replicating it precisely and getting to market faster than competitors. The results validate the precision absorption control of our S-PASS platform.”
석지헌 cake@



![Selbion Up 2 Days, Eyes Lekraza Upside<BR><BR>[[K-Bio Pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/07/PS25072500367b.jpg)

!["'렉라자' 뛰어넘을 자신" 셀비온, 연이틀 상승[바이오맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2025/07/PS25072500558b.jpg)
