Unauthorized reproduction or distribution is illegal and subject to criminal penalties.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
Pharm Edaily enforces a zero-tolerance policy and will take strict action.
This article was released as Pharm Edaily Premium Content on 03/10/2025 10:16:04 AM
Subscribe
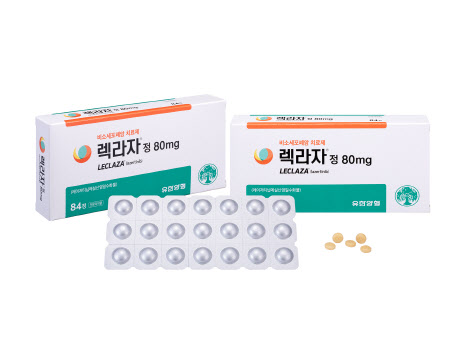
[Kim Jiwan, Edaily Reporter] Oscotec‘s stock price surged following the news of lazertinib’s marketing approval in the UK, marking another milestone after last year‘s approvals in the U.S. and Europe.
Anigen saw its stock price hit the daily limit as the company provided a strong incentive to investors to complete its previously uncertain rights offering, acting as a catalyst for the surge.
Vigencell’s stock price soared after its cell therapy product under development received approval for compassionate use.
According to KG Zeroin MP Doctor (formerly Market Point) on the 7th, Oscotec closed at KRW 31,850, up KRW 2,550 (8.70%) from the previous day. Anigen hit the price ceiling, rising KRW 2,010 to close at KRW 8,730. Vibecell gained 14.19% (KRW 405) to end at KRW 3,260.
Oscotec Gains on lazertinib‘s UK Approval
Oscotec’s stock price surged following the news that Lekraza (ingredient: Lazertinib) received marketing authorization in the UK.
On the 6th (local time), the UK Medicines and Healthcare products Regulatory Agency (MHRA) announced the approval of “Razzcluse” (Lekraza’s brand name in the U.S. and Europe) and Johnson & Johnson (J&J) subsidiary Innovative Medicines (formerly Janssen)’s “Rybrevant” (ingredient: Amivantamab) for combination therapy as a treatment for epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC).
Oscotec is the original developer of Lekraza, an NSCLC treatment. The drug was licensed out to Yuhan Corporation in the preclinical stage in 2015. Later, Yuhan further developed the technology and licensed it to J&J’s Janssen in 2018 for $1.255 billion (KRW 1.6 trillion).
This approval is particularly significant due to the milestone payments tied to Lekraza’s development stages. The revenue from these milestones is shared between Yuhan Corporation and Oscotec at a 60:40 ratio.
Following Lekraza’s FDA combination therapy approval last year, Yuhan received KRW 80 billion (approx. $60 million) in milestone payments, which were distributed between Yuhan and Oscotec based on the agreed 60:40 split.
Oscotec reported KRW 29.1 billion in revenue and KRW 19.6 billion in operating profit for Q3 2023. In contrast, the company‘s 2023 annual revenue stood at KRW 5 billion, with an operating loss of KRW 32.7 billion.
Industry insiders expressed optimism, stating, “Lekraza was approved in Europe at the end of last year, and recently received a recommendation for approval in Japan. In China, approval is expected within the year.”
Anigen Moves Forward with Rights Offering by Slashing New Share Price
Anigen hit the upper limit after increasing the feasibility of its KRW 20 billion rights offering.
Since the end of last year, Anigen had been trying to secure investors for a third-party rights offering but faced difficulties. However, the company successfully turned the situation around by significantly easing investment conditions.
A company representative stated, “The rights offering price now reflects the current market value, which has boosted investor confidence in the offering’s success.”
On the 6th, Anigen issued a revised disclosure regarding its rights offering decision, lowering the new share issuance price from KRW 10,663 to KRW 6,079 while maintaining the total issue amount at KRW 20 billion.
The company initially announced its rights offering on December 20, 2023. However, as the stock price fell below the initially planned issuance price, concerns grew over its feasibility. The revised issuance price has led the market to believe that the rights offering is now more likely to materialize.
Vigencell Soars on Cell Therapy Compassionate Use Approval
Vigencell’s stock price skyrocketed following the compassionate use approval of its cell therapy product.
The company’s cell therapy candidate, VT-Tri, received compassionate use approval from South Korea’s Ministry of Food and Drug Safety (MFDS) on the 6th. Compassionate use approval allows patients with life-threatening or severe conditions who have no other treatment options to access investigational drugs.
Previously, Seoul St. Mary‘s Hospital at The Catholic University of Korea applied for VT-Tri’s compassionate use approval for a patient with acute myeloid leukemia (AML). The approval details are available through MFDS’s Drug Safety Korea portal.
VT-Tri is based on Vigencell’s Vitiar platform, which differentiates and cultures T cells from blood into antigen-specific cytotoxic T cells (CTLs). These CTLs are grown in vitro to recognize and eliminate specific viral and tumor antigens, enhancing their cancer-killing ability.
Vigencell reported that another drug developed using the Vitiar platform, VT-EBV-N, was administered to malignant lymphoma patients and followed up for over five years, showing that over 90% of patients did not experience cancer recurrence.
For AML, a researcher-led clinical study from 2007 to 2013 targeting a single WT1 tumor antigen (WT1-CTL) showed a relapse-free survival rate of 71% and a 0% relapse rate after five years.
VT-Tri is currently undergoing Phase 1, Cohort 2 clinical trials for relapsed/refractory AML. The company secured Phase 1 Investigational New Drug (IND) approval for high-risk AML in 2020.
The approval has boosted expectations as it coincides with the recent revision of the ’Advanced Regenerative Medicine and Advanced Biopharmaceuticals Act‘ (Korean Advanced Regenerative Medicine Act).
On February 24, Vigencell obtained approval for its Cell Processing Facility under the Advanced Biopharmaceutical Good Manufacturing Practice (GMP) regulations from MFDS.
A company representative stated, “With the new regulatory framework in place, the cell therapy sector is expected to benefit significantly. We are preparing to accelerate commercialization and generate revenue from our pipeline.”
|
Vigencell’s stock price soared after its cell therapy product under development received approval for compassionate use.
According to KG Zeroin MP Doctor (formerly Market Point) on the 7th, Oscotec closed at KRW 31,850, up KRW 2,550 (8.70%) from the previous day. Anigen hit the price ceiling, rising KRW 2,010 to close at KRW 8,730. Vibecell gained 14.19% (KRW 405) to end at KRW 3,260.
Oscotec Gains on lazertinib‘s UK Approval
Oscotec’s stock price surged following the news that Lekraza (ingredient: Lazertinib) received marketing authorization in the UK.
On the 6th (local time), the UK Medicines and Healthcare products Regulatory Agency (MHRA) announced the approval of “Razzcluse” (Lekraza’s brand name in the U.S. and Europe) and Johnson & Johnson (J&J) subsidiary Innovative Medicines (formerly Janssen)’s “Rybrevant” (ingredient: Amivantamab) for combination therapy as a treatment for epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC).
Oscotec is the original developer of Lekraza, an NSCLC treatment. The drug was licensed out to Yuhan Corporation in the preclinical stage in 2015. Later, Yuhan further developed the technology and licensed it to J&J’s Janssen in 2018 for $1.255 billion (KRW 1.6 trillion).
This approval is particularly significant due to the milestone payments tied to Lekraza’s development stages. The revenue from these milestones is shared between Yuhan Corporation and Oscotec at a 60:40 ratio.
Following Lekraza’s FDA combination therapy approval last year, Yuhan received KRW 80 billion (approx. $60 million) in milestone payments, which were distributed between Yuhan and Oscotec based on the agreed 60:40 split.
Oscotec reported KRW 29.1 billion in revenue and KRW 19.6 billion in operating profit for Q3 2023. In contrast, the company‘s 2023 annual revenue stood at KRW 5 billion, with an operating loss of KRW 32.7 billion.
Industry insiders expressed optimism, stating, “Lekraza was approved in Europe at the end of last year, and recently received a recommendation for approval in Japan. In China, approval is expected within the year.”
Anigen Moves Forward with Rights Offering by Slashing New Share Price
Anigen hit the upper limit after increasing the feasibility of its KRW 20 billion rights offering.
Since the end of last year, Anigen had been trying to secure investors for a third-party rights offering but faced difficulties. However, the company successfully turned the situation around by significantly easing investment conditions.
|
A company representative stated, “The rights offering price now reflects the current market value, which has boosted investor confidence in the offering’s success.”
On the 6th, Anigen issued a revised disclosure regarding its rights offering decision, lowering the new share issuance price from KRW 10,663 to KRW 6,079 while maintaining the total issue amount at KRW 20 billion.
The company initially announced its rights offering on December 20, 2023. However, as the stock price fell below the initially planned issuance price, concerns grew over its feasibility. The revised issuance price has led the market to believe that the rights offering is now more likely to materialize.
Vigencell Soars on Cell Therapy Compassionate Use Approval
Vigencell’s stock price skyrocketed following the compassionate use approval of its cell therapy product.
The company’s cell therapy candidate, VT-Tri, received compassionate use approval from South Korea’s Ministry of Food and Drug Safety (MFDS) on the 6th. Compassionate use approval allows patients with life-threatening or severe conditions who have no other treatment options to access investigational drugs.
Previously, Seoul St. Mary‘s Hospital at The Catholic University of Korea applied for VT-Tri’s compassionate use approval for a patient with acute myeloid leukemia (AML). The approval details are available through MFDS’s Drug Safety Korea portal.
VT-Tri is based on Vigencell’s Vitiar platform, which differentiates and cultures T cells from blood into antigen-specific cytotoxic T cells (CTLs). These CTLs are grown in vitro to recognize and eliminate specific viral and tumor antigens, enhancing their cancer-killing ability.
|
Vigencell reported that another drug developed using the Vitiar platform, VT-EBV-N, was administered to malignant lymphoma patients and followed up for over five years, showing that over 90% of patients did not experience cancer recurrence.
For AML, a researcher-led clinical study from 2007 to 2013 targeting a single WT1 tumor antigen (WT1-CTL) showed a relapse-free survival rate of 71% and a 0% relapse rate after five years.
VT-Tri is currently undergoing Phase 1, Cohort 2 clinical trials for relapsed/refractory AML. The company secured Phase 1 Investigational New Drug (IND) approval for high-risk AML in 2020.
The approval has boosted expectations as it coincides with the recent revision of the ’Advanced Regenerative Medicine and Advanced Biopharmaceuticals Act‘ (Korean Advanced Regenerative Medicine Act).
On February 24, Vigencell obtained approval for its Cell Processing Facility under the Advanced Biopharmaceutical Good Manufacturing Practice (GMP) regulations from MFDS.
A company representative stated, “With the new regulatory framework in place, the cell therapy sector is expected to benefit significantly. We are preparing to accelerate commercialization and generate revenue from our pipeline.”





![i-Sens Soars 23% on EU CGM Expansion [K-bio pulse]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26021300327b.jpg)

![양동원 서울성모병원 부원장 “레켐비·슈퍼브레인 병용, 치매 진행 지연 효과”[전문가 인사이트]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26021700064b.jpg)
![냉탕 온탕 오간 에이프릴바이오…실적 호조에 로킷·휴젤 상승[바이오맥짚기]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26021200275b.jpg)
